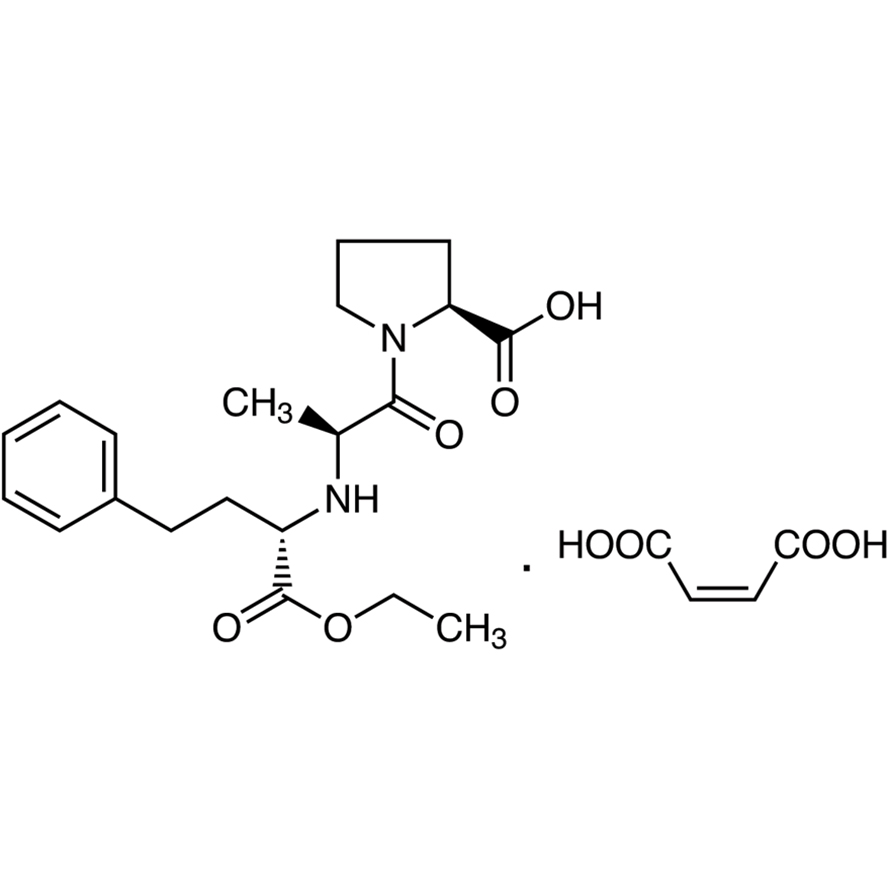
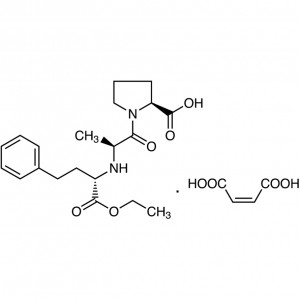
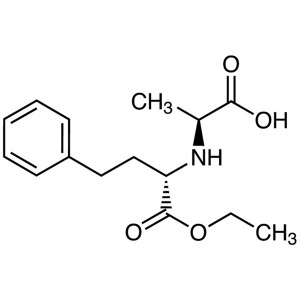
Enalapril Maleate CAS 76095-16-4 Assay 98.0~102.0% API High Purity
Manufacturer Supply Enalapril Maleate Intermediate With High Purity
N-[(S)-1-Ethoxycarbonyl-3-phenylpropyl]-L-alanine; ECPPA CAS: 82717-96-2
Enalapril Maleate CAS: 76095-16-4
| Chemical Name | Enalapril Maleate |
| Synonyms | MK-421; 1-[N-[(S)-1-Ethoxycarbonyl-3-phenylpropyl]-L-alanyl]-L-proline Maleate |
| CAS Number | 76095-16-4 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C24H32N2O9 |
| Molecular Weight | 492.52 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Crystalline Powder |
| Identification A | The IR spectrum matches that of RS |
| Identification B | Major peak in chromatogram obtain in the test solution in the assay accordance with that of reference solution |
| Specific Rotation | -41.0° ~ -43.5.0° |
| Loss on Drying | ≤1.0% |
| Residue on Ignition | ≤0.20% |
| Heavy Metals | ≤10ppm |
| Related Substances | |
| Enalaprilat | ≤0.30% |
| Moexipril Related Compound F | ≤0.30% |
| Enalapril Cyclohexyl Analog | ≤0.30% |
| Enalapril Related Compound D | ≤0.30% |
| Any Unspecified Impurity | ≤0.10% |
| Total Impurities | ≤2.00% |
| Residual Solvents | |
| Ethanol | ≤5000ppm |
| Acetone | ≤5000ppm |
| Dichloromethane | ≤600ppm |
| n-Hexane | ≤290ppm |
| Assay | 98.0%~102.0% (Calculated on the dried basis) |
| Test Standard | USP Standard; EP Standard; Enterprise Standard |
| Usage | Active Pharmaceutical Ingredient (API) |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry and well-ventilated warehouse away from incompatible substances. Protect from light and moisture. Store away from oxidizing agents.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Enalapril Maleate
C20H28N2O5·C4H4O4 492.52
l-Proline, 1-[N-[1-(ethoxycarbonyl)-3-phenylpropyl]-l-alanyl]-, (S)-, (Z)-2-butenedioate (1:1).
1-[N-[(S)-1-Carboxy-3-phenylpropyl]-l-alanyl]-l-proline 1'-ethyl ester, maleate (1:1) [76095-16-4].
Enalapril Maleate contains not less than 98.0 percent and not more than 102.0 percent of C20H28N2O5·C4H4O4, calculated on the dried basis.
Packaging and storage-Preserve in well-closed containers, and store at controlled room temperature.
USP Reference standards <11>-
USP Enalapril Maleate RS Click to View Structure
Identification-
A: Infrared Absorption <197M>.
B: The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Specific rotation <781S>: between -41.0 and -43.5.
Test solution: 10 mg per mL, in methanol.
Loss on drying <731>-Dry it in vacuum at a pressure not exceeding 5 mm of mercury at 60 for 2 hours: it loses not more than 1.0% of its weight.
Residue on ignition <281>: not more than 0.2%.
Heavy metals, Method II <231>: 0.001%.
Related compounds-
pH 6.8 Phosphate buffer, pH 2.5 Phosphate buffer, Solution A, Solution B, Mobile phase, Diluent, Enalapril diketopiperazine solution, System suitability solution, and Chromatographic system— Proceed as directed in the Assay.
Standard solution-Dissolve an accurately weighed quantity of USP Enalapril Maleate RS in Diluent, and dilute quantitatively, and stepwise if necessary, with Diluent to obtain a solution having a known concentration of about 3 µg per mL.
Test solution-Use the Assay preparation.
Procedure-Separately inject equal volumes (about 50 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak area responses. Calculate the percentage of each impurity in the portion of Enalapril Maleate taken by the formula:
100(CS / CT)(ri / rS)
in which CS is the concentration, in mg per mL, of USP Enalapril Maleate RS in the Standard solution; CT is the concentration, in mg per mL, of Enalapril Maleate in the Test solution; ri is the peak area of each impurity obtained from the Test solution; and rS is the peak area of enalapril obtained from the Standard solution: not more than 1.0% of any impurity having a relative retention time of about 1.10 is found; not more than 0.3% of any other individual impurity is found; and not more than 2% of total impurities is found.
Assay-
pH 6.8 Phosphate buffer-Dissolve 2.8 g of monobasic sodium phosphate in about 900 mL of water in a 1000-mL volumetric flask. Adjust with a 9 M sodium hydroxide solution to a pH of about 6.8, dilute with water to volume, and mix.
pH 2.5 Phosphate buffer-Dissolve 2.8 g of monobasic sodium phosphate in about 900 mL of water in a 1000-mL volumetric flask. Adjust with phosphoric acid to a pH of about 2.5, dilute with water to volume, and mix.
Solution A-Prepare a filtered and degassed mixture of pH 6.8 Phosphate buffer and acetonitrile (19:1).
Solution B-Prepare a filtered and degassed mixture of acetonitrile and pH 6.8 Phosphate buffer (33:17).
Mobile phase-Use variable mixtures of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography 621).
Diluent-Prepare a mixture of pH 2.5 Phosphate buffer and acetonitrile (95:5).
Enalapril diketopiperazine solution-Carefully place about 20 mg of USP Enalapril Maleate RS in a 100-mL beaker to form a mound on the bottom of the beaker. Place the beaker on a hot plate at about one-half the maximum hot plate temperature setting. Heat for about 5 to 10 minutes until the solid is melted. Immediately remove the beaker from the hot plate, and allow to cool. [note-Avoid overheating to prevent heat-induced degradation, which would give rise to a brown color.] To the cooled residue in the beaker add 50 mL of acetonitrile, and sonicate for a few minutes to dissolve. The solution typically contains, in each mL, between 0.2 mg and 0.4 mg of enalapril diketopiperazine.
Standard preparation-Dissolve an accurately weighed quantity of USP Enalapril Maleate RS in Diluent, and dilute quantitatively, and stepwise if necessary, with Diluent to obtain a solution having a known concentration of about 0.3 mg per mL.
System suitability solution-Add 1 mL of Enalapril diketopiperazine solution to a 50-mL portion of the Standard preparation, and mix.
Assay preparation- Transfer about 30 mg of Enalapril Maleate, accurately weighed, to a 100-mL volumetric flask, dissolve in and dilute with Diluent to volume, and mix.
Chromatographic system (see Chromatography <621>)-The liquid chromatograph is equipped with a 215-nm detector and a 4.1-mm × 15-cm column that contains packing L21. The flow rate is about 1.5 mL per minute. The column temperature is maintained at 70. The chromatograph is programmed as follows.
Time(minutes) Solution A(%) Solution B(%) Elution
0 95 5 equilibration
0-20 95→40 5→60 linear gradient
20-25 40 60 isocratic
25-26 40→95 60→5 linear gradient
26-30 95 5 isocratic
Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention times are about 1.0 for enalapril and 2.1 for enalapril diketopiperazine; and the resolution, R, between enalapril and enalapril diketopiperazine is not less than 3.5. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 1.0%.
Procedure- Separately inject equal volumes (about 50 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C20H28N2O5·C4H4O4 in the portion of Enalapril Maleate taken by the formula:
100C(rU / rS)
in which C is the concentration, in mg per mL, of USP Enalapril Maleate RS in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.

Risk Codes R36/37/38 - Irritating to eyes, respiratory system and skin.
R62 - Possible risk of impaired fertility
R63 - Possible risk of harm to the unborn child
Safety Description S22 - Do not breathe dust.
S24/25 - Avoid contact with skin and eyes.
S36/37 - Wear suitable protective clothing and gloves.
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
UN IDs 3077
WGK Germany 2
RTECS TW3666000
HS Code 2933990099
Toxicity LD50 oral in rat: 2973mg/kg
Enalapril Maleate (CAS: 76095-16-4) is an angiotensin converting enzyme inhibitor (ACE), used to treat high blood pressure, diabetes kidney disease, and chronic heart failure. Orally active. An Antihypertensive agent. Enalapril maleate (Vasotec), the active metabolite of enalapril, competes with angiotensin I for binding at the angiotensin-converting enzyme, blocking the conversion of angiotensin I to angiotensin II.